Guide FDA Packaging And Labeling Requirements

July 18, 2024 Box Customization
It is crucial for businesses that deal in food, and drugs also including cosmetic sectors to be capable of understanding the complex running and the numerous regulations set by the FDA regarding packaging and labeling.
The Food and Drug Administration performs a very crucial and significant role in consumer protection and enforcing companies’ information disclosure.
By reading this guide you will be able to find out more details about the model of custom CBD packaging and labeling laws including the legal provisions related to labeling the essentials of a label along with how to ensure compliance with these laws.
These are crucial to understanding by firms as a way of being in compliance with the laws and ensuring every consumer that their products are safe to use.
FDA Labeling Requirements for Food
Federal labeling laws for foods are well developed and exhaustively championed to increase the credibility of the information passed to the public and buyers of foods.
Below of some of the areas that the FDA legislation for labeling food covers.
Nutrition Labeling
Federal regulations are required to include a panel labeled as Nutrition Facts with serving sizes and product content including calories and nutrients accompanied by the %DV.
Ingredient Declaration
The very important must be declared on the label in the order of their proportion in the product with a trend towards.
The lowest additives that do not have a significant impact on the quality of food and milk products as preservatives must be accompanied by the names used in everyday language.
Allergen Labeling
These lists must be specific and point out major food allergens like milk, and eggs along with peanuts or in a statement contains.
Net Quantity (in grams) of Contents
The net quantity of contents label must mention the actual quantity of products in the packets through metric and US standard measurements to make the customers aware of how much product they are going to consume.
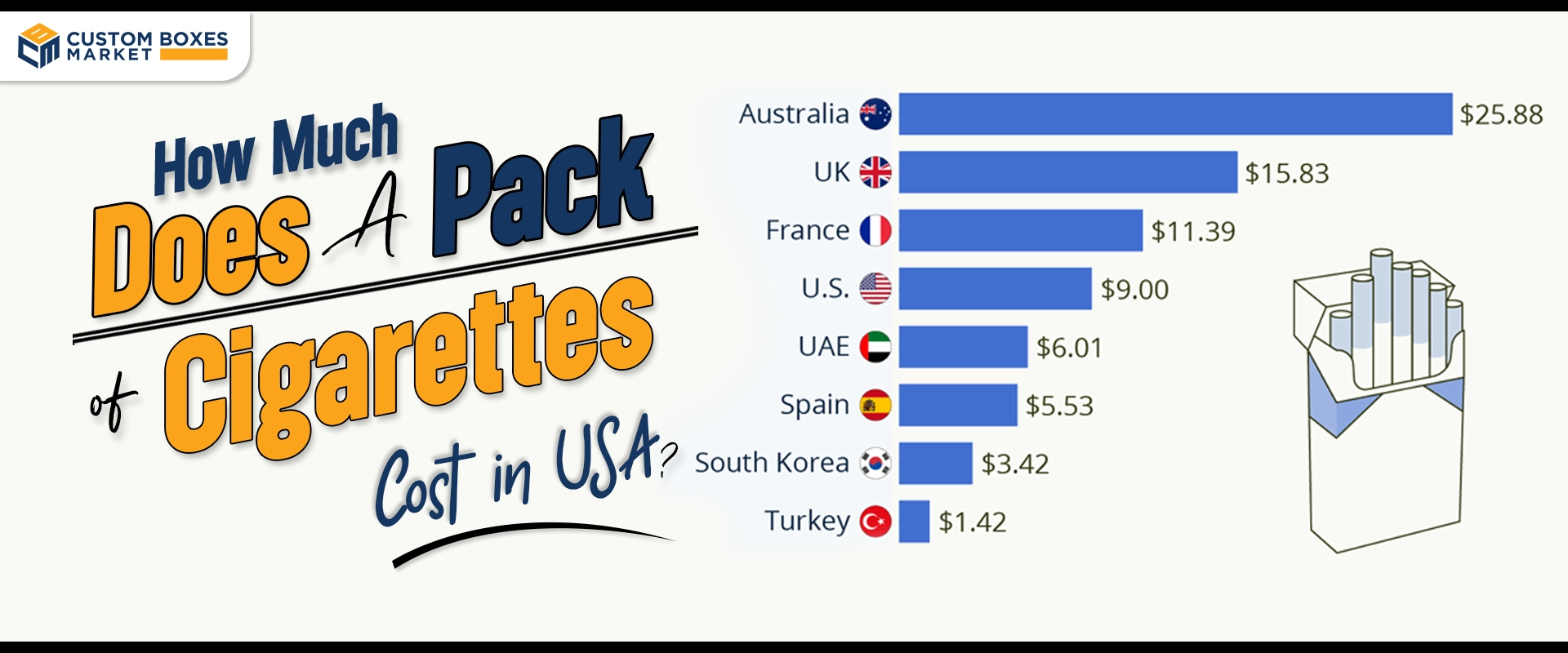
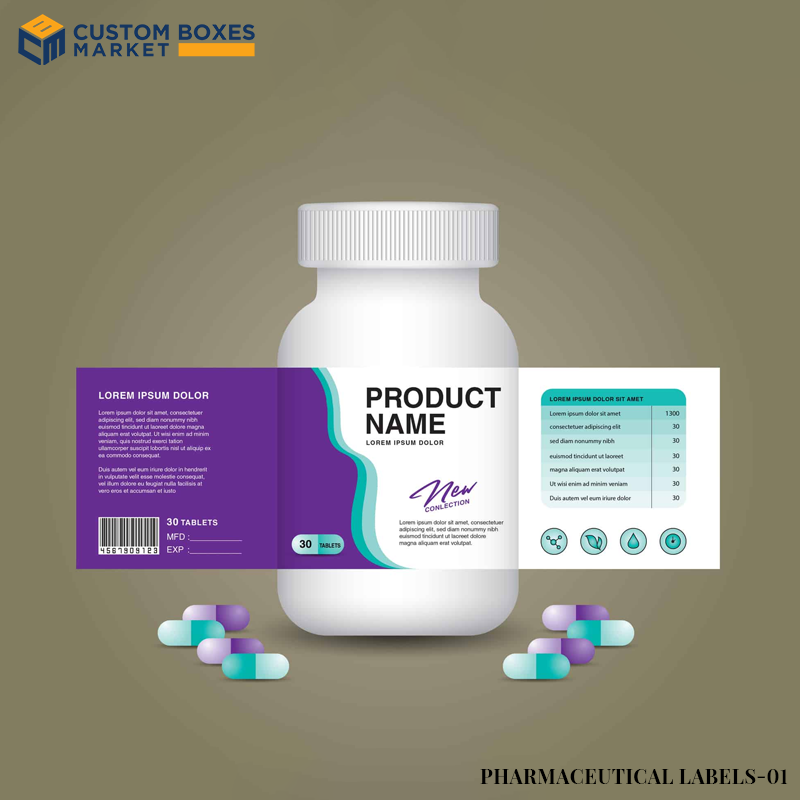
FDA Labeling Requirements for Drugs
There are labels known as standard labels used by the FDA on drugs to ensure that particular drug labels fulfill the requirements for the specific category of drug labels.
Safeguard the lives of the patients and provide required information to the health care practitioners and the patient customers.
Below, is a brief overview of the FDA’s current labeling requirements as it relates to drug products.
External Labeling Primary Display Panel
Therefore according to the FDA, the PDP of prescription drugs must accompany pharmacological information concerning the particular drug this information shall include the brand name of the drug the ingredients strength dosage form along quantity.
Drug Facts Labeling
Most of the OTC drug labels need to include a Drug Facts panel that contains information about how the drug works.
Its purpose is the intended uses to be taken precautions as well as directions on how to use the drug among other relevant information that is useful for the safe use of the drug.
Prescription Labeling
Prescription labels should contain some details for the concerned prescription and these details are the name of the drug, power of the drug purpose administration precaution to be followed expiry date as well as the details of the pharmacist.
Child-Resistant Packaging
It is widely known from the basket literature that certain drugs especially those that are so toxic that they should not be ingested at all will require extra special care to help avoid situations.
Where children accidentally take the product by opening the packages In this regard, the FDA has recommended that such products should be packed in child-resistant containers.
FDA Labeling Requirements for Cosmetics
Corresponding to visions of Occupy for Beauty FDA labeling rules for cosmetics are geared towards providing the buyers with the needed information about the commodity they are likely to buy and to protect them.
Below are some of the significant elements that the FDA has placed on the labeling of cosmetics.
Product Identity
This makes it mandatory for cosmetics to be labeled with their chemical name to let the buyer know the kind of product that they are using.
Quantity of Substance per Container
This must be displayed together with the common name to enable the consumer to determine or estimate the amount of product in terms of weight measure or several items within the package.
Ingredient Declaration
They must be declared on the labels depending on the quantity so that consumers easily distinguish between products with ingredients that may cause allergies or affect their skin.
Manufacturer Information
The label must also contain the name and the place of business of the manufacturer packer or distributor. This way a consumer can communicate with whoever he or she may want to ask questions to report a problem with the product.
FDA Labeling for Medical Devices
Present labeling requirements set by the Food and Drug Administration for medical devices are essential at this present time because they safeguard patients from receiving and using low-quality medical products.
Play a very significant role in offering information to healthcare practitioners and other consumers in society.
Finally, here are the headlines of the FDA labeling standards for medical devices.
Device Identification
Labels of medical devices need to have the name or identifier of the device its model or catalog number and other information to facilitate the identification of the device and its parts.
Intended Use
This could include intended use or IFU intended patient population or diagnosis and other items as needed.
Instructions for Use
Proper usage and safety guidelines are to be communicated when it comes to handling the device and its components assembling its functioning as well as the precautions that need to be taken when using the device.
Manufacturer Information
The Custom labels should declare the person who manufactured the device the packer or distributor company’s name physical address and other necessary means of contact.
Best Practices for Compliance
Certainly! Here are samples of effective tips for compliance.
Stay Updated on Regulations
The new regulation should also include periodic reviews of updates and changes in the current and existing regulations such as that of the FDA.
Here you could subscribe to the regulatory newsletters attend conferences and be engaged in industry associations.
Implement Robust Documentation Processes
It also requires every organization to retain full documentation of compliance activities policies and procedures as well as the records of regulatory activities.
Organize business document workflows so that personnel follow the same procedures and have an accountable way to track document ownership and changes.
Training and Education
Make sure to educate them on what is considered compliance needs actions as well as expected behaviors.
Education should be done continuously by our staff since there are changes in laws and policies that affect the various positions they hold.
Risk Assessment and Management
Schedule compliance risk evaluation as frequently as possible to be able to contemplate the compliance risks that may exist and tackle them in the proper sequence.
To manage risks you need to adapt them to your organization’s activities and its sphere of responsibilities and rules.
Internal Audits And Monitoring
It is essential to perform studies internally to ensure that guidelines are being followed and policies put in place are suitable.
To correct any shortcomings and findings of the problem to be able to locate places that need correction and correction then be done accordingly.
Seek the Services of Regulatory Agencies
Phone contacts with regulatory authorities particularly the FDA should be established and cleared with the latter when there is the need to seek clarification.
The inspected school is obliged to answer the inquiries notifications and findings of the inspection promptly to show the willingness to cooperate and adhere to the guidelines.
Conclusion
In conclusion, guiding FDA packaging and labeling requirements can seem complex, but it doesn’t have to be.
By introducing yourself to the regulations and pursuing guidance from experts like CBM, you can ensure your products are compliant and safe for consumers, and avoid costly delays.
It is worth identifying some benefits of proper and transparent labeling it helps the consumers to make their choices consciously and enhances the trust in the quality of the product.
Through active regulation awareness, good quality control mechanisms and structures and constant engagement with the regulation authorities, firms are in a position to present a good example of how they will uphold the spirit of safety and good standards.
Categories
Recent Posts
19 / February , 2026 Box Customization
16 / February , 2026 Box Material
3 / February , 2026 Gift Boxes
26 / January , 2026 Gift Boxes
6 / January , 2026 Sustainable Packaging
3 / December , 2025 Packaging Stats
17 / November , 2025 Custom Packaging
14 / November , 2025 Product By Industry
4 / November , 2025 Shipping Protection
28 / October , 2025 Custom Packaging
9 / October , 2025 Custom Packaging
7 / October , 2025 Shipping Protection
24 / September , 2025 Custom Packaging
Get Free Quote
Related Products